Cyclophane
Recent Related Publications

Mixed Amide Paracyclophane Assemblies Emulating Supramolecular Copolymers. Cole D. Stearns,† Ajeet Kumar,† Ion Ghiviriga, Lukasz M. Dobrzycki, Khalil A. Abboud, and Ronald K. Castellano*. J. Am. Chem. Soc. 2025, 147, 24615.
Go to article
Tuning Supramolecular Polymer Assembly through Stereoelectronic Interactions. Will R. Henderson, Guancen Liu, Khalil A. Abboud, and Ronald K. Castellano*. J. Am. Chem. Soc. 2021, 143, 12688.
Go to articleSupramolecular polymerization of chiral molecules devoid of chiral centers. Will R Henderson, and Ronald K Castellano*. Polym Int 2021; 70: 897.
Go to article
Influence of Amide Connectivity on the Hydrogen-Bond-Directed Self-Assembly of [n.n]Paracyclophanes. Will R. Henderson, Ajeet Kumar, Khalil A. Abboud, and Ronald K. Castellano*. Chem. Eur. J. 2020, 26, 17588.

Self-Assembling [n.n]Paracyclophanes: A Structure–Property Relationship Study. Will R. Henderson, Yu Zhu, Danielle E. Fagnani, Guancen Liu, Khalil A. Abboud, and Ronald K. Castellano*. J. Org. Chem. 2020, 85, 1158.
Go to articleDesign and Synthesis of New Therapeutics
The “weak” inter- and intramolecular interactions that underlie synthetic supramolecular chemistry are also fundamental to enzyme structure, function, and targeting. Over the past several years we have collaborated with researchers in the UF College of Medicine to design and study, using a physical organic approach, novel therapeutics (as antihypertensives and anticancer agents). Some work has shown how small-molecule activation of endogenous Angiotensin Converting Enzyme 2 (ACE2) attenuates, and even reverses, hypertension-linked pathophysiology in vivo. More recent work has introduced Disulfide Bond Disrupting Agents (DDAs) as a unique class of drugs to combat breast cancer tumorigenesis.
Recent Related Publications

Non-Canonical, Strongly Selective Protein Disulfide Isomerases as Anticancer Therapeutic Targets. Mary E. Law,† Zaafir M. Dulloo,† Brian Hardy, Ania Kelegama, Reagan Clark, Mariana Rivas Montbrun, Gabriella Antmann, Srihith Nooka, Ronald K. Castellano, and Brian K. Law*. Biomolecules 2025, 15, 1146.
Go to article
DR5 Disulfide Bonding Functions as a Sensor and Effector of Protein Folding Stress. ME Law,† ZM. Dulloo,† SR Eggleston, GP Takacs, GM Alexandrow, Y il Lee, M Wang, B Hardy, H Su, B Forsyth, P Das, PK Datta, CW Chiang, A Sharma, SRR Kanumuri, OA Guryanova, JK Harrison, B Tirosh, RK Castellano*, and BK Law*. Mol. Cancer Res. (2025) 23 (7): 622.
Go to article
Beta-cyclodextrin formulation of a disulfide-bond disrupting agent for improved systemic exposure. Zaafir M. Dulloo, Ion Ghiviriga, Mary E. Law, Sarvesh K. Verma, Abhisheak Sharma, Brian K. Law*, and Ronald K. Castellano*. RSC Med. Chem. 2025, 16, 3622.
Go to articleInhibitors of ERp44, PDIA1, and AGR2 induce disulfide-mediated oligomerization of Death Receptors 4 and 5 and cancer cell death. Mary E Law, Elham Yaaghubi, Amanda F Ghilardi, Bradley J Davis, Renan B Ferreira, Jin Koh, Sixue Chen, Sadie F DePeter, Christopher M Schilson, Chi-Wu Chiang, Coy D Heldermon, Peter Nørgaard, Ronald K Castellano*, and Brian K Law*. Cancer Letters 534 (2022) 215604.
Go to articleRepurposing Tranexamic Acid as an Anticancer Agent. Mary E. Law, Bradley J. Davis, Amanda F. Ghilardi, Elham Yaaghubi, Zaafir M. Dulloo, Mengxiong Wang, Olga A. Guryanova, Coy D. Heldermon, Stephan C. Jahn, Ronald K. Castellano, and Brian K. Law*. Front. Pharmacol. 2022, 12, 792600.
Go to articleAnticancer Agents Derived from Cyclic Thiosulfonates: Structure-Reactivity and Structure-Activity Relationships. Amanda F. Ghilardi,† Elham Yaaghubi,† Renan B. Ferreira, Mary E. Law, Yinuo Yang, Bradley J. Davis, Christopher M. Schilson, Ion Ghiviriga, Adrian E. Roitberg, Brian K. Law*, and Ronald K. Castellano*. ChemMedChem 2022, 17, e202200165.
Go to articleSymmetrical Molecular Scaffolds for Rapid Functionalization
We have made synthetic and mechanistic contributions to the design of molecular scaffolds that allow the rapid and efficient preparation of discrete multifunctional architectures. Seminal work involves benzotrifuranone (BTF), a highly symmetric trilactone capable of one-pot sequential aminolysis to afford a trifunctionalized product. It joins only one other molecular reagent capable of useful single-pot trifunctionalization.
Learn MoreRecent Related Publications
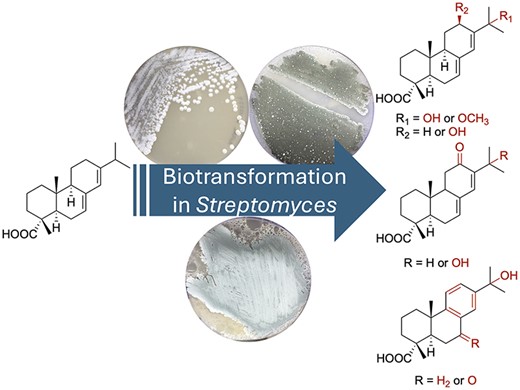
McCadden, C. A.; et al. Biocatalytic diversification of abietic acid in Streptomyces. J. Ind. Microbial. Biotechnol. 2025, 52, kuaf003.
Go to article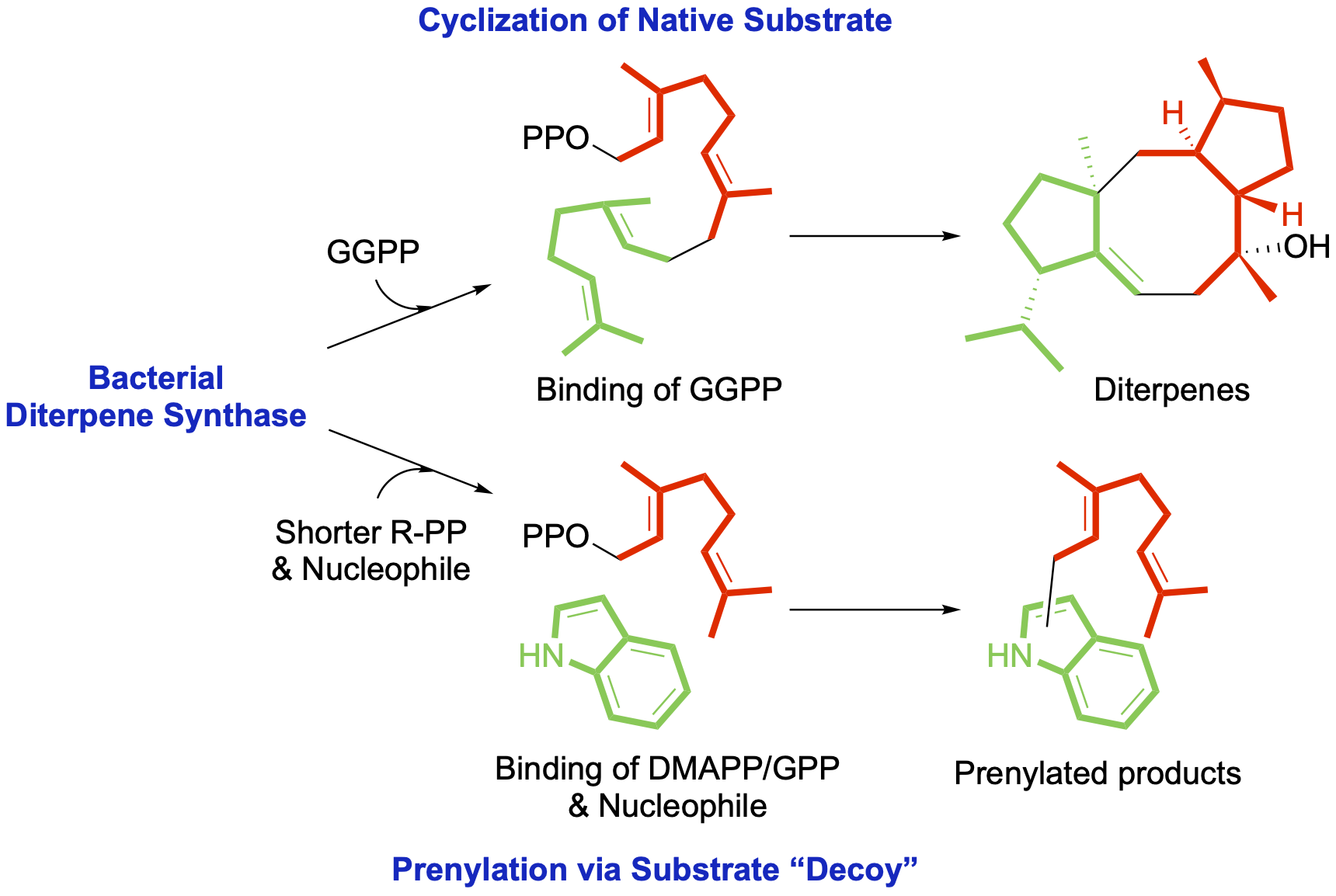
Xu, B.; et al. Mutation of the eunicellane synthase Bnd4 alters its product profile and expands its prenylation ability. Org. Biomol. Chem. 2022, 20, 8833–8837.
Go to articleScience is always most fun with friends! Combining knowledge, skills, and viewpoints often leads to a deeper understanding of the science and the most impactful findings.
Recent Related Publications
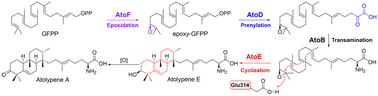
Wang, Z.; et al. Biosynthesis of a bacterial meroterpenoid reveals a non-canonical class II meroterpenoid cyclase. Chem. Sci. 2025, 16, 310–317.
Go to article
Chen, X.; et al. Canonical terpene synthases in arthropods: Intraphylum gene transfer. Proc. Natl. Acad. Sci. 2024, 121, e2413007121.
Go to article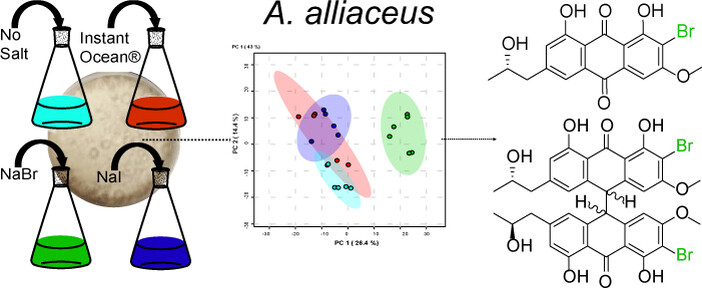
Mandelare, P. E.; et al. Chemical diversity of Aspergillus alliaceus phenotypes: discovery of brominated bianthrones with activity against triple-negative breast cancer cell lines. ChemBioChem. 2024, 25, e202400398.
Go to article